
FHH promotes innovation by introducing state-of-art machines in Egyptian market
Fine Hygienic Holding (FHH), one of the world’s leading wellness groups and manufacturers of hygienic ...
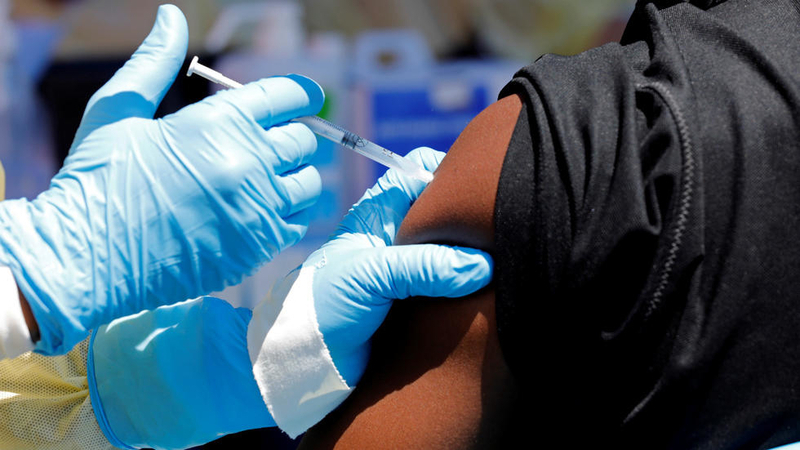
MSF will manage the new vaccine’s implementation in collaboration with DRC’s Ministry of Health and the Institut National de Recherche Biomedicale. Additionally, MSF community engagement teams have been meeting with people in Majengo and Kahembe health districts in Goma to share information about the vaccine and answer their questions. These two districts have been identified in collaboration with health authorities as the first to be offered the vaccine.
Vaccination is one of the key tools to protect people from the Ebola virus. Merck’s rVSV-ZEBOV vaccine—the vaccine currently being used most widely in DRC—has been proven to be effective and has become a valuable resource in the fight against the epidemic. The introduction of a second vaccine is not meant to replace the rVSV vaccine, but to complement it and hopefully provide us with an additional tool in the fight against future Ebola outbreaks. As this is the tenth outbreak of Ebola in DRC, it makes sense to work towards having another vaccine option introduced into the country.
The vaccine was endorsed in the interim recommendations by WHO’s Strategic Advisory Group of Experts (SAGE) on immunization in May of 2019. It was chosen because it has the most potential among other candidate vaccines. Also because it had already passed phase I and II clinical trials, with over 6,000 human volunteers participating. These studies showed that the vaccine has an excellent safety profile.
Fine Hygienic Holding (FHH), one of the world’s leading wellness groups and manufacturers of hygienic ...
Foodics, the leading provider of restaurant management and financial tech solutions in the MENA region, ...
Banque Misr copped the title of the best CSR initiative prize of the MEED MENA ...


اترك تعليقا